Our Technology
Foresight CLARITY™ is powered by our proprietary PhasED-Seq™ ctDNA technology
PhasED-Seq™ was developed by oncologists who were dissatisfied with the detection limit of other circulating tumor DNA (ctDNA) assays, particularly at post-treatment landmark timepoints when ctDNA levels are lowest .1

SEE LEVELS BELOW 1 PART PER MILLION
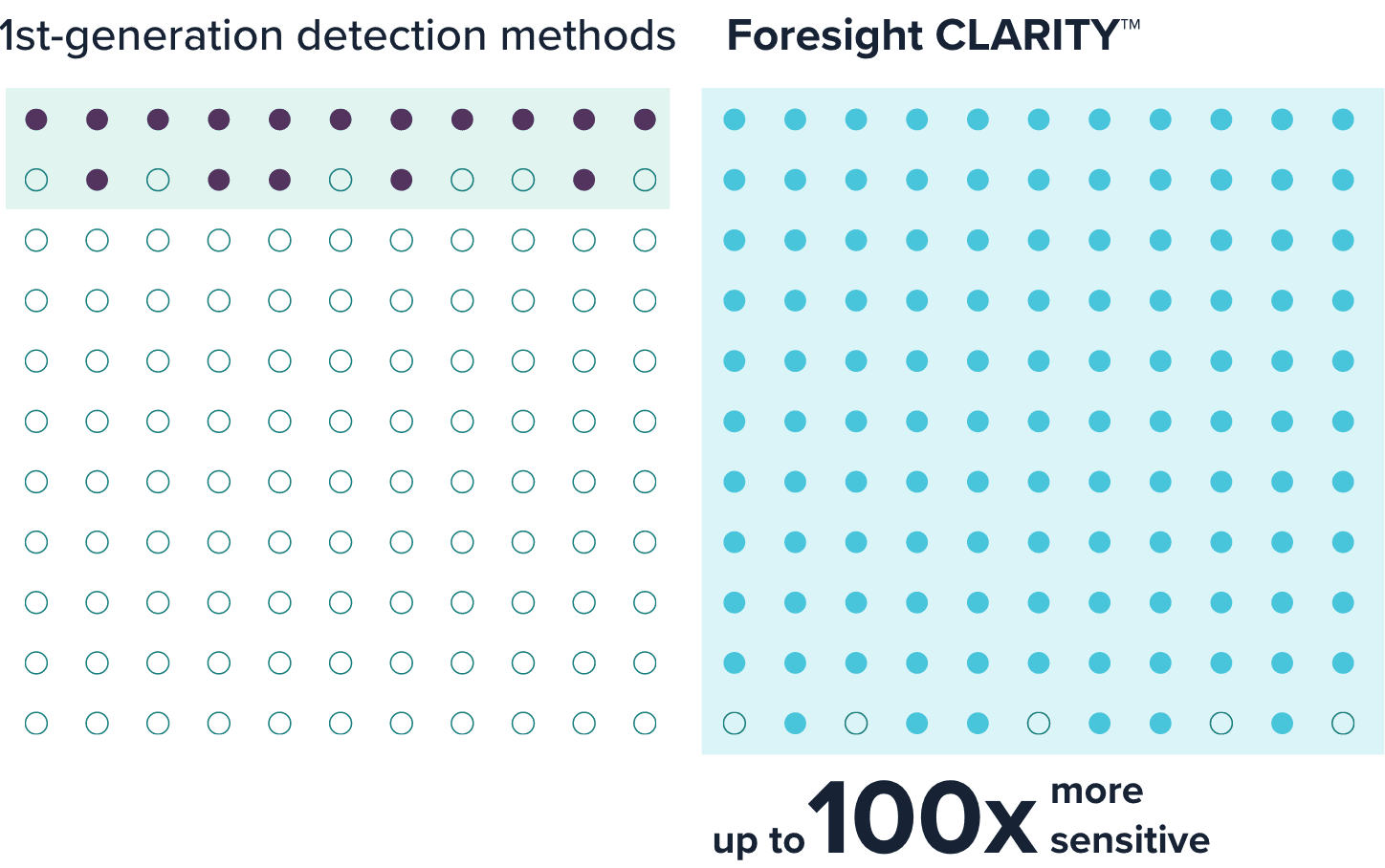
By tracking unique genomic differences between cancer and healthy cells with unparalleled accuracy, Foresight CLARITY™ can detect ctDNA levels below one part per million, which is up to 100 times more sensitive than other first-generation liquid biopsy methods.1,14,16
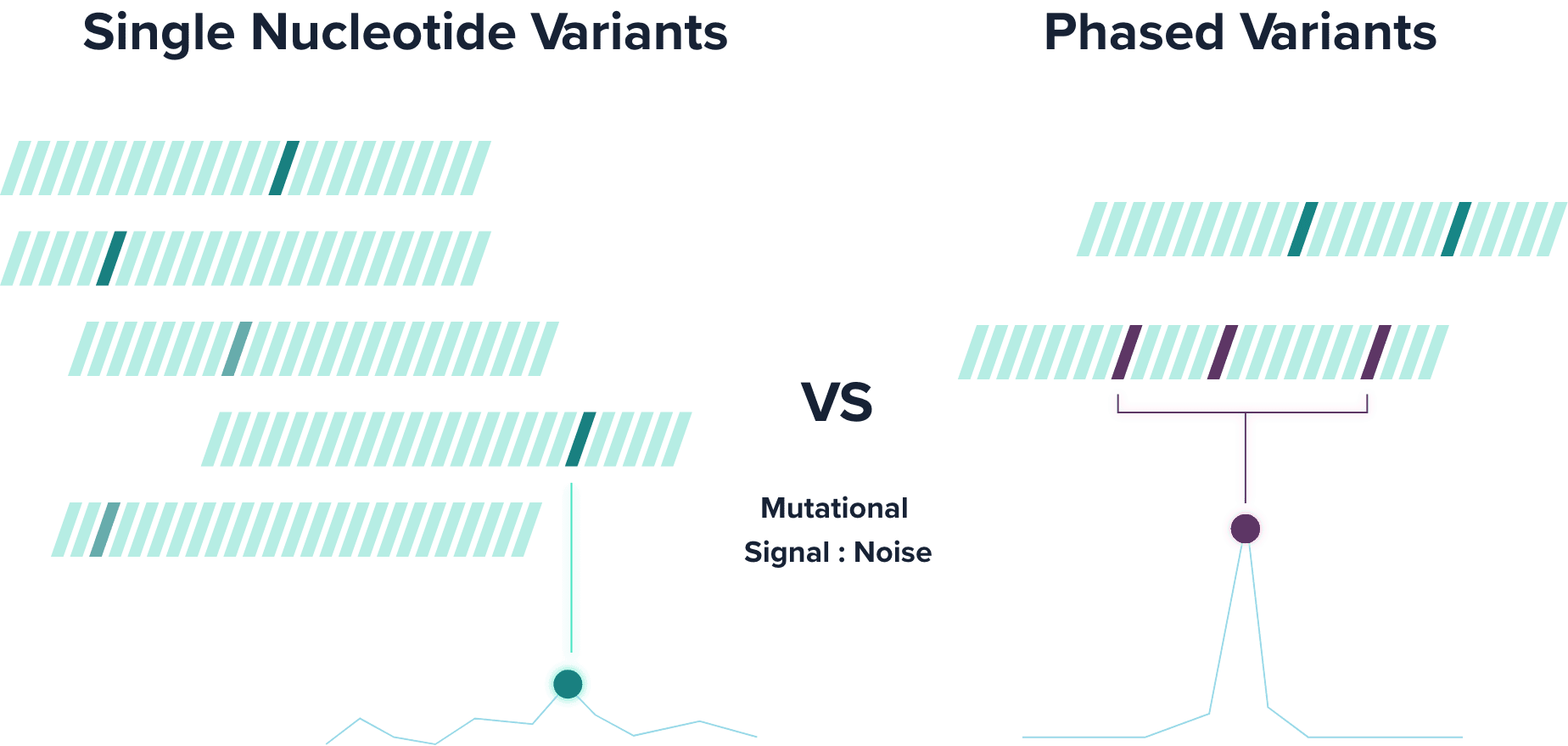
PhasED-Seq™ tracks phased variants (PVs), which are two or more single nucleotide variants (SNVs) that occur on the same DNA molecule. Leveraging PVs to detect ctDNA substantially reduces the background error rate compared to traditional SNV-based approaches.
Greater confidence in diagnostic data
The accuracy and performance of Foresight CLARITY™ ensure the highest confidence in your research and clinical development programs.
Reduce ‘noise’ in sequencing data to decrease false negative and false positive rates
Strengthen hazard ratios and better delineate the effect of treatment
Screen fewer patients to achieve the same or higher statistical power of study
Learn more about Foresight CLARITY™ MRD, powered by PhasED-Seq™.


- Kurtz DM, Soo J, Co Ting Keh L, et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol. 2021;39(12):1537-1547. https://doi.org/10.1038/s41587-021-00981-w