Products & Services
Foresight CLARITY™ MRD Platform
Early and accurate relapse detection
Foresight CLARITY™, powered by PhasED-Seq™ technology, is a liquid-biopsy platform designed to confidently detect the presence of minimal residual disease (MRD) from circulating tumor DNA (ctDNA) with unprecedented sensitivity across multiple cancers.
By detecting recurrence with greater accuracy than imaging and first-generation assays, we hope to enable clinically-actionable decisions at key points in the patient journey that can deliver the greatest impact.1,2,3


In large B-cell lymphoma, Foresight CLARITY™ detected relapse
200 days earlier than PET/CT1
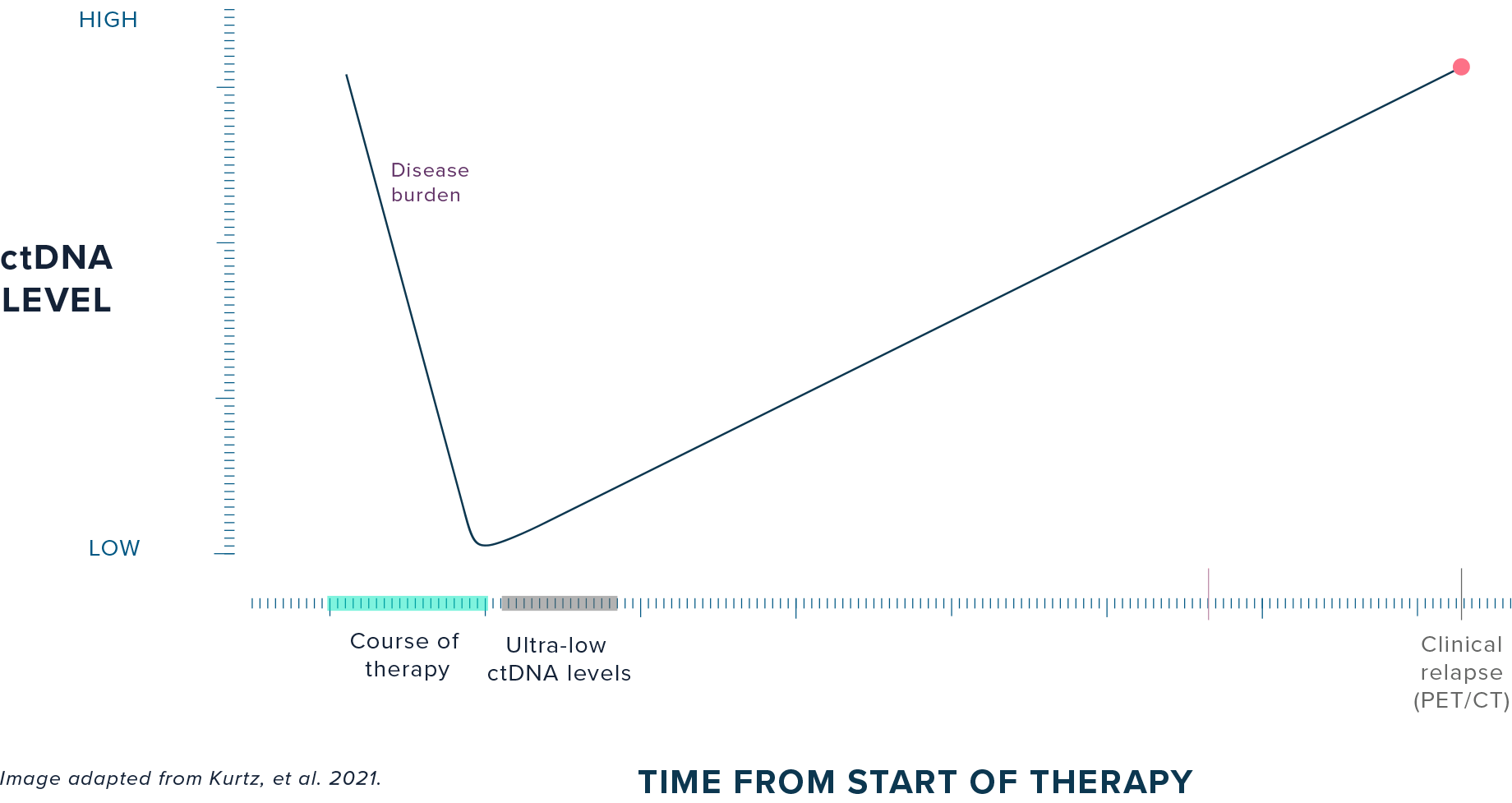
PATIENT HEALTH JOURNEY:
Disease burden typically declines during the course of therapy, but residual disease may remain at ultra-low levels at the end of treatment despite radiographic clearance. While 30-40% of DLBCL patients relapse following 1L treatment, it may take one to two years to clinically diagnose relapse with PET/CT (standard of care).4
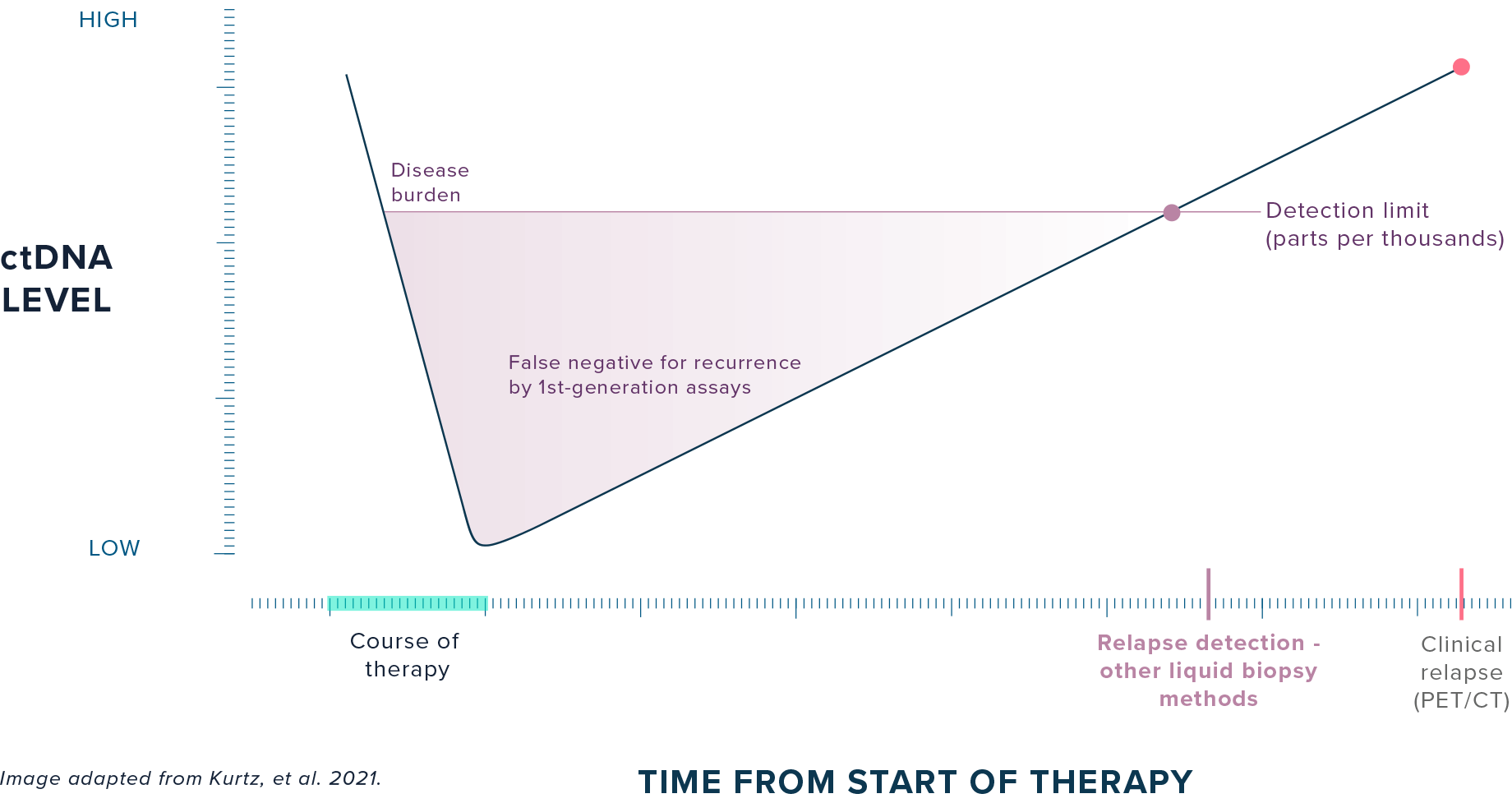
CURRENT DETECTION METHODS:
First-generation liquid biopsy methods lack the sensitivity to detect ultra-low levels of residual disease remaining at the end of treatment, preventing clinicians from intervening with continued therapy.
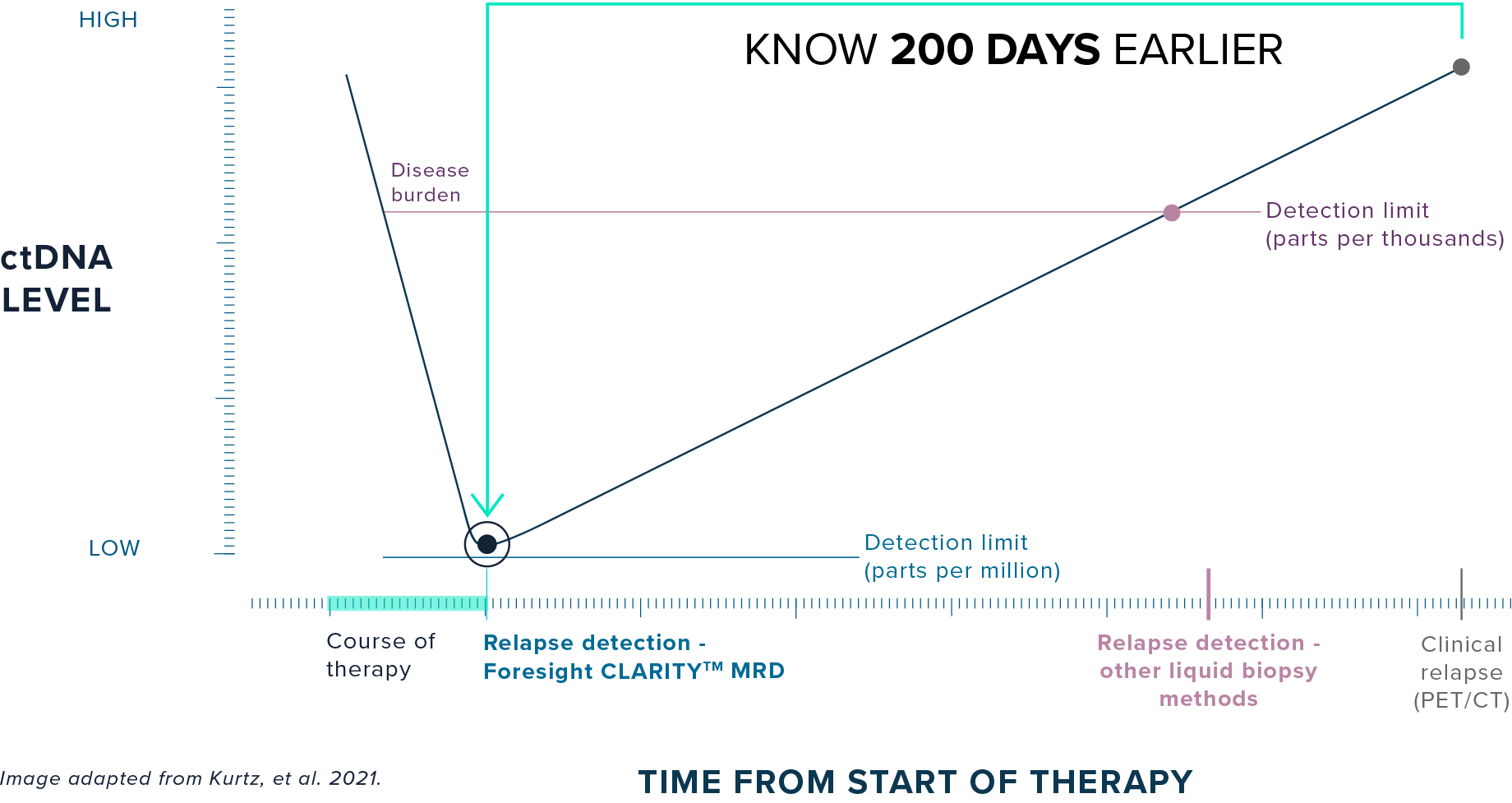
FORESIGHT CLARITY™:
Foresight CLARITY™ detects ultra-low ctDNA levels immediately at the end of treatment, potentially allowing clinicians to treat patients while disease burden is low and intervene 200 days earlier than imaging-based relapse detection.
See clearly. Act decisively.
Using MRD for accurate and early response assessment has the opportunity to change current treatment paradigms and enable actionable therapeutic decision-making at landmark timepoints:
FORESIGHT CLARITY™ FOR:
B-cell Lymphoma
Foresight CLARITY™ for B-cell Lymphoma delivers earlier and more accurate response assessment than standard-of-care PET/CT imaging through ultra-sensitive detection of MRD.1,7,8,9
Guidelines for B-cell Lymphoma now recommend the utilization of a ctDNA-MRD assay with an analytical sensitivity of <1ppm to adjudicate patients who are PET positive after frontline therapy.17
FEATURES:
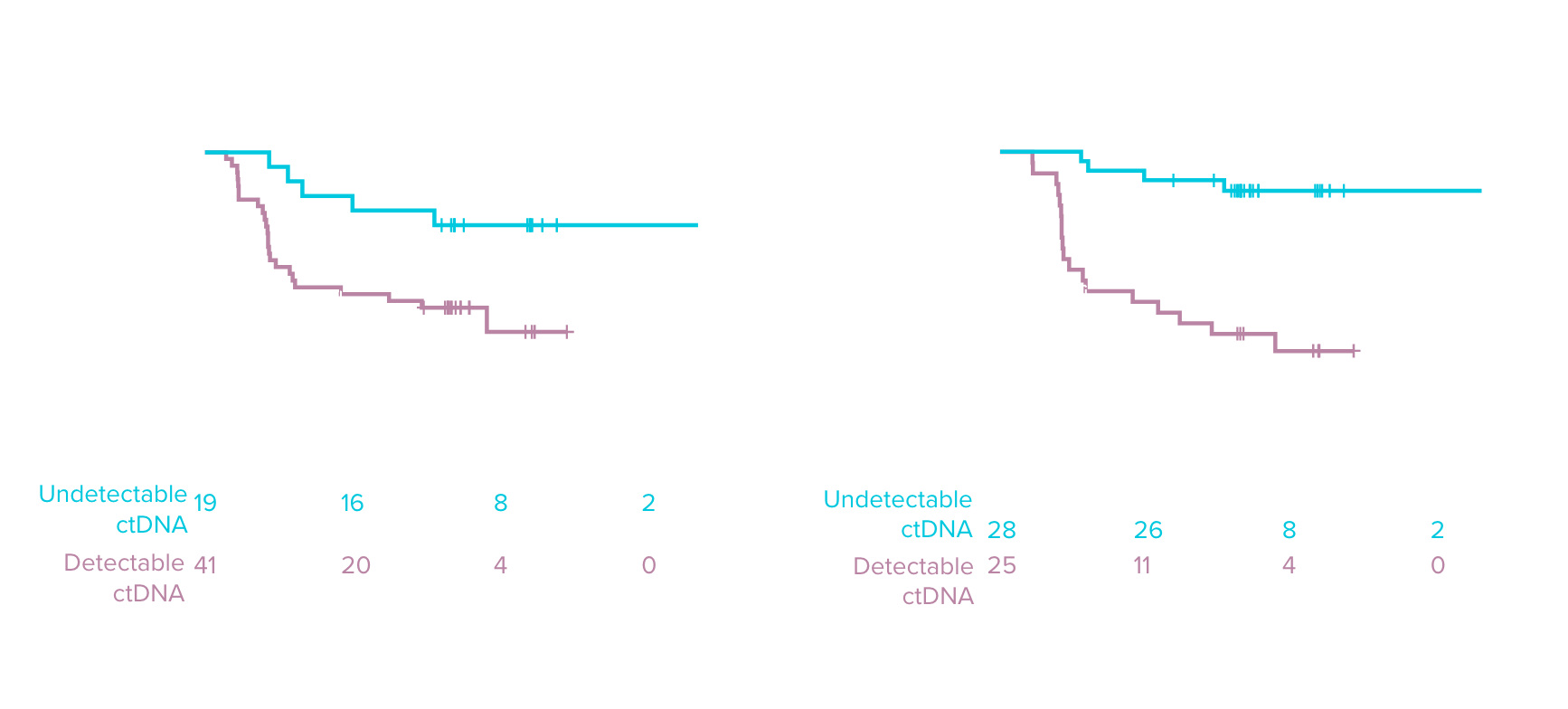
Detection limit in DLBCL1,16
Clinical sensitivity in DLBCL at EOT1,3,7
Analytical specificity in DLBCL at EOT 16
Fixed, off-the-shelf assay for fast turnaround from our central CLIA-registered lab
Phased variants determined from either pre-treatment plasma or tumor tissue sample
Post-treatment MRD timepoints measured from standard blood draw volumes
INDICATIONS:
- DLBCL/LBCL
- Follicular lymphoma
- Classic Hodgkin lymphoma
- Additional hematological cancer indications available for exploratory research. Please contact us.
In studies comparing MRD and PET/CT at END OF TREATMENT, Foresight CLARITY™ demonstrated the ability to:
Offer improved prognostic value in cases with discordant MRD and PET/CT at EOT7
Prognostic performance of MRD in cases with discordant ctDNA-MRD and PET/CT at end of the treatment showed that MRD was more accurate of the two. This suggests an opportunity to use MRD to adjudicate imaging for response assessment.
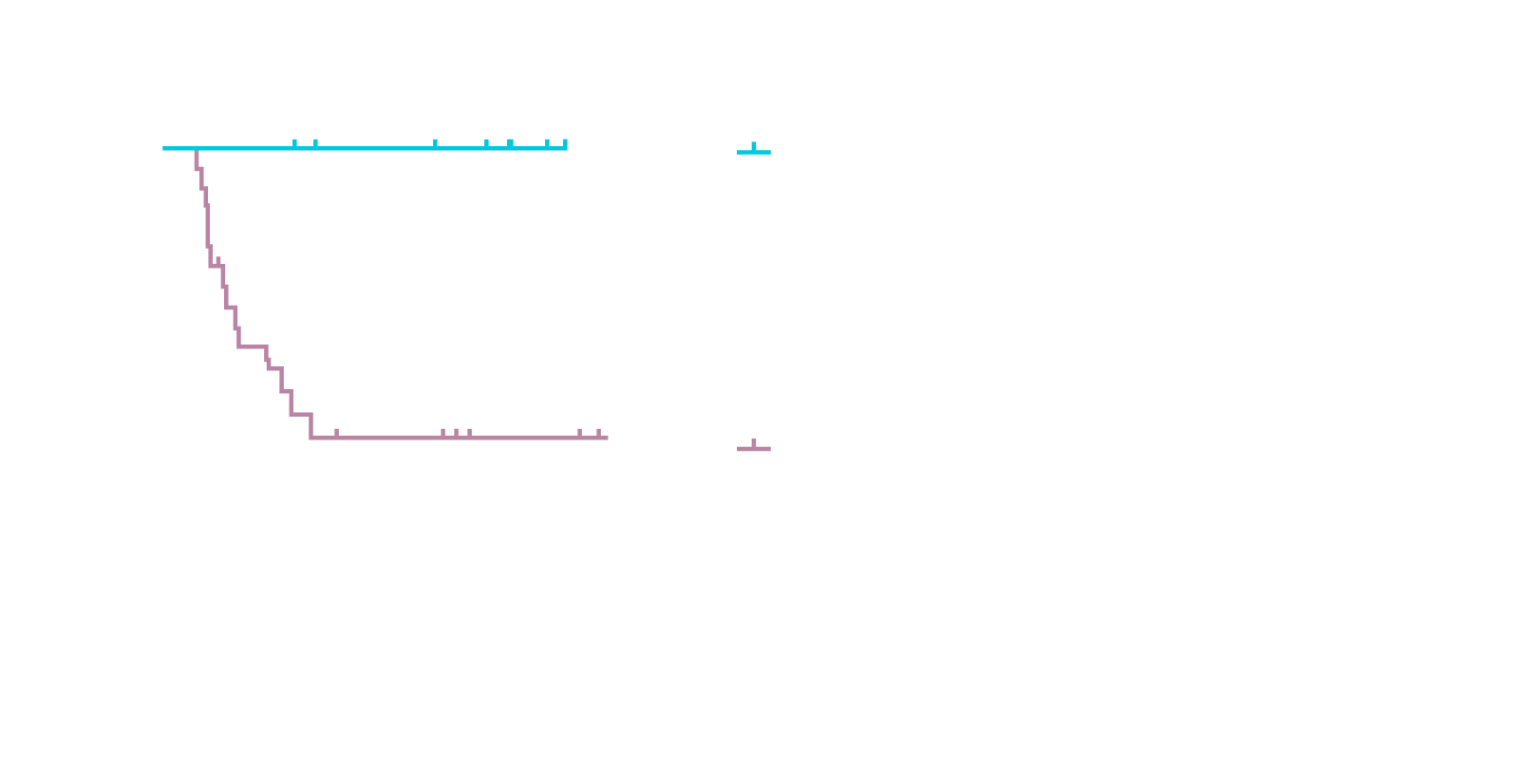
Accurately predict deep, durable therapeutic response as early as 15 days after CAR-T infusion12
Undetectable ctDNA after liso-cel treatment strongly correlated with longer EFS at mid-treatment time points, pointing to the opportunity for MRD to be an early indicator of therapeutic efficacy in trials.
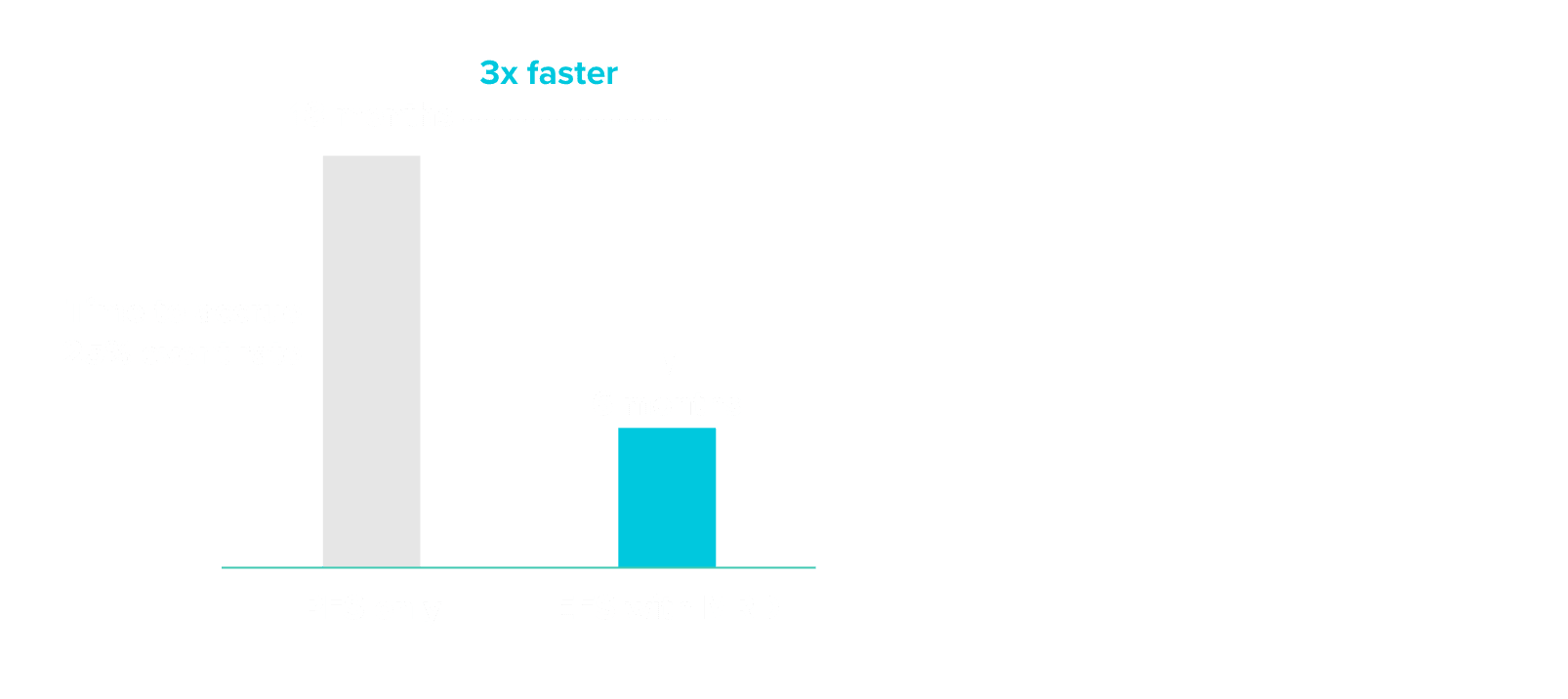
Reduce clinical trial timelines by up to 12 months as a surrogate endpoint to PFS9
A simulation analysis using data from multiple LBCL cohorts shows that using event-free survival with MRD status could have shortened clinical trial timelines by 12 months.
Incorporating MRD as an endpoint may accelerate typical clinical trial readout timelines, potentially up to 3x faster.
FORESIGHT CLARITY™ FOR:
Solid Tumors
Studies show that Foresight CLARITY™ for Solid Tumors delivered earlier and more accurate response assessment than first-generation liquid biopsy methods.2,11,13
FEATURES:
Detection limit in NSCLC11
Improved clinical sensitivity at post-surgical landmark in NSCLC vs. first-generation SNV methods11
Personalized panel
Accepts plasma from standard blood draw volumes
INDICATIONS:
- NSCLC
- Breast Cancer
- Additional solid tumor indications available for exploratory research. Please contact us for more information.
In studies comparing ctDNA detection by Foresight CLARITY™ vs. SNV-based methods, Foresight CLARITY™ demonstrated the ability to:
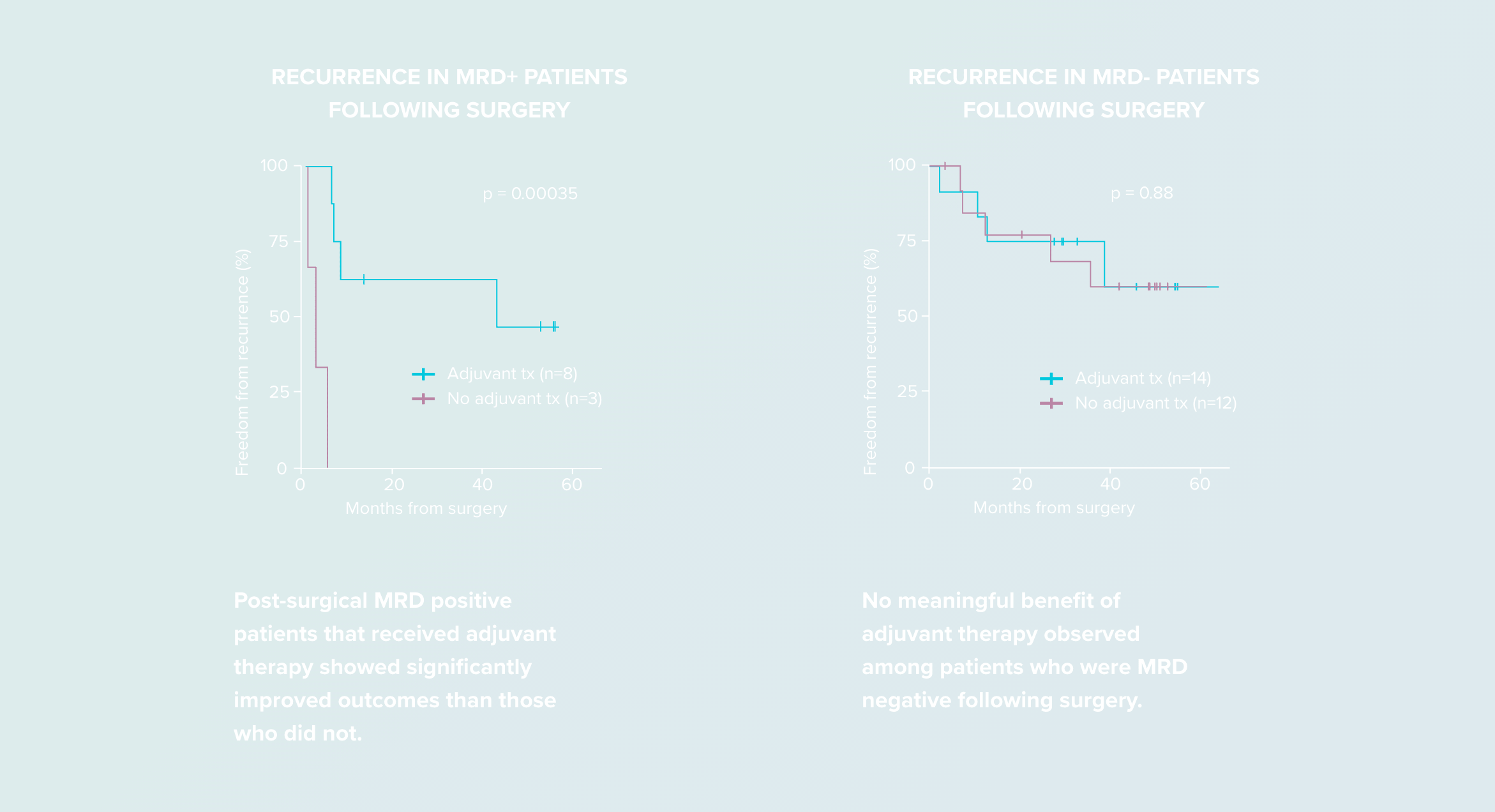
Identify high-risk post-surgical patients who may benefit from adjuvant therapy11
References:
- Kurtz DM, Soo J, Co Ting Keh L, et al. Enhanced detection of minimal residual disease by targeted sequencing of phased variants in circulating tumor DNA. Nat Biotechnol. 2021;39(12):1537-1547. https://doi.org/10.1038/s41587-021-00981-w
- Isbell JM, Li BT, Eichholz JE, et al. Ultrasensitive ctDNA MRD monitoring in early stage lung cancer with PhasED-Seq™. Poster presented at: American Association for Cancer Research (AACR); 2023. (See poster)
- Roschewski M, Kurtz DM, Westin J, et al. MRD-negativity after frontline DLBCL therapy: pooled analysis of 6 clinical trials. Abstract presented at: International Conference on Malignant Lymphoma (ICML); June 14, 2023. https://doi.org/10.1002/hon.3163_112
- Nuvvula S, Dahiya S, Patel SA. The novel therapeutic landscape for relapsed/refractory diffuse large B cell lymphoma. Clin Lymphoma Myeloma Leuk. 2022;22(6):362-372. https://doi.org/10.1016/j.clml.2021.11.010
- Roschewski M, Phelan JD, Pittaluga S, et al. Phase 2 study of acalabrutinib window prior to frontline therapy in untreated aggressive B-cell lymphoma: preliminary results and correlatives of response to acalabrutinib. Blood. 2021;138(Supplement):524-524. https://doi.org/10.1182/blood-2021-145228
- Westin J, Steiner RE, Chihara D, et al. 856 Smart stop: lenalidomide, tafasitamab, rituximab, and acalabrutinib alone and with combination chemotherapy for the treatment of newly diagnosed diffuse large B-cell lymphoma. Presented at: American Society of Hematology (ASH); 2023. https://doi.org/10.1182/blood-2023-180381
- Sworder BJ, Yoon SE, Kim SJ, et al. Prognostic utility of minimal residual disease (MRD) after curative intent induction therapy for DLBCL: a prospective real-world ctDNA study. Presented at: American Society of Hematology (ASH); 2023. (See presentation)
- Roschewski M, Lindenberg L, Mena E, et al. End of treatment response assessment after frontline therapy for aggressive B-cell lymphoma: landmark comparison of a singular PET/CT scan vs ultrasensitive circulating tumor DNA. Presented at: American Society of Hematology (ASH); 2023. (See presentation)
- Goldstein J, Kim SW, Yoon SE, et al. Optimizing circulating tumor DNA limits of detection for DLBCL during first line therapy. Blood. 2023;142(suppl 1):187. (See presentation)
- Allogene Therapeutics – ALPHA3 Phase 2 Trial.
- Isbell JM, Goldstein JS, Hamilton EG, et al. Ultrasensitive circulating tumor DNA (ctDNA) minimal residual disease (MRD) detection in early stage non-small cell lung cancer (NSCLC). Poster presented at: American Society of Clinical Oncology (ASCO); 2024. (See poster)
- Stepan L, Ansari S, Okal A, et al. Circulating tumor DNA dynamics as early outcome predictors for lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma from the phase 3 TRANSFORM study. Blood. 2023;142(suppl 1):225. https://doi.org/10.1182/blood-2023-181007
- Kurtz DM, Chabon JJ, Sworder B, et al. Leveraging phased variants for personalized minimal residual disease detection in localized non-small cell lung cancer. Poster presented at: American Society of Clinical Oncology (ASCO); 2021. (See poster)
- Cabel L, Ah-Reum An J, Kurtz D, et al. Ultra-sensitive ctDNA detection and monitoring in early breast cancer using PhasED-Seq. ESMO Congress 2024; Barcelona, Spain (See poster)
- Kurtz DM, Hogan GJ, Schultz A, et al. Ultrasensitive MRD profiling predicts outcomes in DLBCL after frontline therapy with tafasitamab in combination with lenalidomide and R-CHOP. Blood 2022; 140 (Supplement 1): 3498–3499. https://doi.org/10.1182/blood-2022-168378
- Klimova N., Close S., Kurtz D. M., Hockett R. D., Hyland L. Analytical validation of a circulating tumor DNA assay using PhasED-Seq technology for detecting residual disease in B-cell malignancies. Oncotarget. 2025; 16: 329-336. https://doi.org/10.1101/2024.08.09.24311742
- National Comprehensive Cancer Network. nccn.org