Products & Services
For Biopharma
The next generation of clinical development with ultra-sensitive MRD detection
We partner with innovative biopharma companies at the forefront of leveraging minimal residual disease (MRD) technology for therapeutic development and response assessment. Our products and expert services are tailored to support the entire continuum of drug development, from translational research through clinical trials and diagnostic applications.


Advance your clinical development with CLARITY
Diagnostic Development:
Partner alongside Foresight’s multi-disciplinary team of bioinformatics, lab, medical, and regulatory professionals for diagnostic development
Leverage Foresight’s state-of-the-art, CLIA-certified lab for centralized testing and diagnostic commercialization
Identify patient populations with a higher likelihood of future relapse
Leverage ultra-sensitive ctDNA minimal residual disease (MRD) detection to assess response at key landmark timepoints to select high-risk patient populations and guide treatment
MRD-Driven Clinical Trials
Integrate ctDNA-driven strategies seamlessly into your clinical trial with our CLIA-certified central lab and team of experienced diagnostic, regulatory, and clinical experts
Use Foresight CLARITY™ ultra-sensitive MRD detection as an enrollment biomarker to find more patients and accelerate clinical trial enrollment
Leverage Foresight CLARITY™ ultra-sensitive MRD detection as a surrogate endpoint to accrue response events earlier than PFS/OS and accelerate drug approval and launch timelines
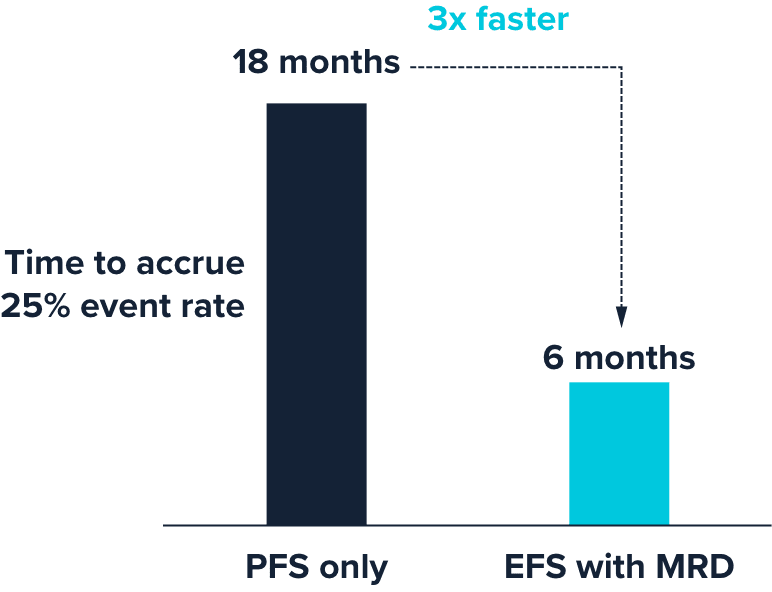
Accelerate clinical trials by Up to 12 months with earlier endpoint measurement
Across multiple studies, Foresight CLARITY™ MRD detection accurately stratified patient response at end of treatment.1,2
Event-free survival (EFS) with ultra-sensitive MRD may accelerate clinical trial readouts by up to a year vs. progression-free survival alone.3
Exploratory Research
Analyze the dynamics of ctDNA and MRD before, during, and after treatment for response assessment and dose optimization
Develop personalized medicine strategies using Foresight CLARITY™ MRD for cohort selection
Gain new insights with early signals of patient response to new therapies
Discover our recent exploratory research with biopharma partners.
Interested in working with Foresight to accelerate drug development timelines?
Contact us to discuss your program.
- Sworder BJ, Yoon SE, Kim SJ, et al. Prognostic utility of minimal residual disease (MRD) after curative intent induction therapy for DLBCL: a prospective real-world ctDNA study. Presented at: American Society of Hematology (ASH); 2023.
- Roschewski M, Kurtz DM, Westin J, et al. MRD-negativity after frontline DLBCL therapy: pooled analysis of 6 clinical trials. Abstract presented at: International Conference on Malignant Lymphoma (ICML); June 14, 2023. https://doi.org/10.1002/hon.3163_112
- Goldstein J, Kim SW, Yoon SE, et al. Optimizing circulating tumor DNA limits of detection for DLBCL during first line therapy. Blood. 2023;142(suppl1):187. https://doi.org/10.1182/blood-2023-187759